strontium electron configuration|electron config strontium : Tagatay Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the . Tingnan ang higit pa Cardholder Guide. Official travel for the Department of Defense just became easier with the Citi Department of Defense Travel Card. When you are preparing to use your new card, please read What To Do When I First Receive My New Card.For more information regarding your new card, please read the Department of Defense Cardholder Guide.. Department .9 talking about this. follow me
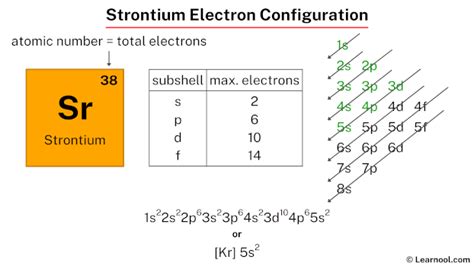
strontium electron configuration,The ground state electron configuration of strontium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s2. This electron configuration shows that the last shell of strontium has two electrons. Therefore, the valence electrons of strontiumare two. The elements that have 1, 2, or 3 electrons in the last shell . Tingnan ang higit pa
The total number of electrons in strontium is thirty-eight. These electrons are arranged according to specific rules in different . Tingnan ang higit pastrontium electron configurationAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the . Tingnan ang higit paelectron config strontiumScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in a certain circular path. These circular . Tingnan ang higit pastrontium electron configuration electron config strontium Mar 23, 2023
In order to write the Sr electron configuration we first need to know the number of electrons for the Sr atom (there are 38 electrons). When we write the .
Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. .Strontium is an alkaline earth metal with symbol Sr and atomic number 38. Its electron configuration is [Kr] 5s 2, which means it has two valence electrons in the outermost shell.Strontium. Full electron configuration of strontium: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s2. rubidium ← strontium → yttrium. Strontium, complete electron configuration. Learn how to determine the arrangement of electrons in strontium atom using different methods, such as aufbau principle, periodic table, Bohr model, and orbital diagram. See examples, . Electron configuration for Sr is termed as the distribution of electrons in its orbits. Strontium consists of 38 electrons. Electron configuration of Strontium is given as below: 1s 2 2s 2 2p 6 3s 2 3p .Electron Dot Model. Chemical Properties of Strontium. Electrochemical Equivalent: 1.635g/amp-hr; Electron Work Function: 2.59eV; Electronegativity: 0.95 (Pauling); 0.99 .Strontium - Properties, history, name origin, facts, applications, isotopes, electronic configuation, crystal structure, hazards and more; Interactive periodic table of the .
Electron shell configuration: [Kr]5s^2; Melting Point (K): 1042; Boiling Point(K): 1657; . Strontium-90, a radioactive isotope of the metal produced by fission reactions is a dangerous environmental menace because its chemistry is similar to calcium and it may take its place in bones. The strong radiation emitted by the isotope interferes .Get the facts about element Strontium (Sr) [38] from the periodic table. Find physical data, electron configuration, chemical properties, aggregation states, isotope data (including decay trees) as well as some historic information.
Orbital diagram. Strontium electron configuration. ← Electronic configurations of elements. Sr (Strontium) is an element with position number 38 in the periodic table. Located in the V period. Melting point: 769 ℃. Density: 2.63 g/cm 3 . Electronic configuration of the Strontium atom in ascending order of orbital energies: 1s 2 2s 2 .Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and .

Strontium (Sr) Strontium is the 38th element in the periodic table and has a symbol of Sr and atomic number of 38. It has an atomic weight of 87.62 and a mass number of 88. Strontium has thirty-eight protons and fifty neutrons in its nucleus, and thirty-eight electrons in five shells. It is located in group two, period five and block s of the .Shell structure: 2,8,18,8,2. Electron configuration: [Kr]5s2. Oxidation state: 2. Crystal structure: cubic. A soft, silvery element that tarnishes in air, strontium is commonly found in nature combined with other compounds, but never as the free element. Strontium reacts vigorously with water and its salts ignite spontaneously in air, imparting .

The electronic configuration of Strontium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s2. What is the abbreviated electronic configuration of Strontium? The abbreviated electronic configuration of Strontium is [Kr] 5s2. To form abbreviated notation of electronic configuration, the completely filled subshells are replaced by the noble gas . To write the configuration for the Strontium ions, first we need to write the electron configuration for just Strontium (Sr). We first need to find the numb.
strontium electron configuration|electron config strontium
PH0 · strontium electron configuration full
PH1 · full electron configuration of platinum
PH2 · electron configuration for every element
PH3 · electron configuration for dummies
PH4 · electron configuration chart pdf
PH5 · electron configuration chart
PH6 · electron configuration calculator
PH7 · electron config strontium
PH8 · Iba pa